|
Quantity
|
Out of stock
|
||
|
|
|||
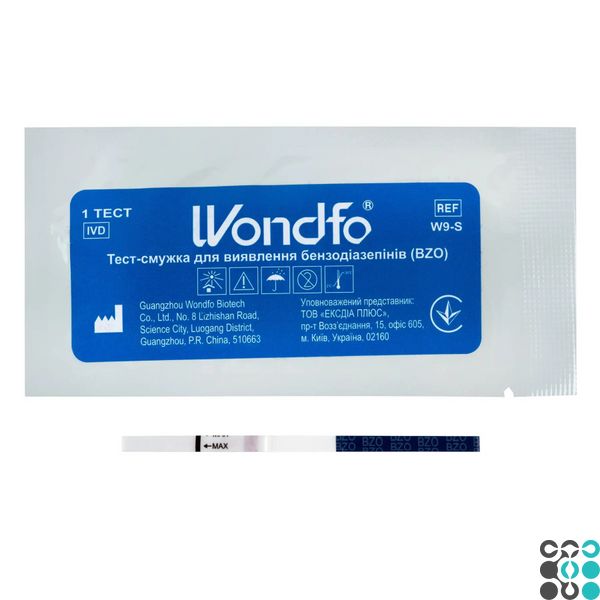
The Wondfo® Benzodiazepine Identification Test (BZO) is a rapid, one-step test for the qualitative determination of benzodiazepines and their main metabolites in human urine at a defined threshold concentration.
For in vitro diagnosis only. For use by healthcare professionals only.
INTENT
The Wondfo® Benzodiazepine Detection Test (BZO) is a flow-through lateral immunochromatographic assay for the determination of benzodiazepines in human urine at a threshold concentration of 300 ng/ml.
BRIEF DESCRIPTION
Benzodiazepines are the most widely used anxiolytic drugs. They are widely used as sedatives, hypnotics, muscle relaxants and anticonvulsants. Used orally or sometimes by injection, they have a wide range of half-lives from 2 to 40 hours. Benzodiazepines are usually detected within 1 to 2 days of their use. Benzodiazepines are metabolized in the liver. Some benzodiazepines and their metabolites are excreted in the urine. Their use may cause drowsiness and/or confusion. Benzodiazepines potentiate the effects of alcohol and other CNS depressants.
Psychological and physical dependence on benzodiazepines can develop as a result of using high doses of the drug for a long period of time.
More...
PRECAUTIONS
This test is for external use only. Do not swallow.
2. Dispose of after the first use. The test must not be used more than once.
3 Do not use the test kit after the expiration date.
4. Do not use the kit if the package is damaged or poorly sealed.
5. Keep out of the reach of children.
6. Do not evaluate results later than 10 minutes.
ASSAY KIT
One package contains the test and a moisture absorber.
The moisture absorber is for storage only and is not used in the analysis.
STORAGE AND STABILITY
Store at 2 ° C - 30 ° C in a closed container until expiration date.
Store in a place protected from direct sunlight, moisture and high temperature.
Do not freeze.
SAMPLE COLLECTION AND PREPARATION
Collect urine sample into a urine cup (container). Urine samples can be stored in the refrigerator (2 °C - 8 °C) for up to 48 hours. For longer storage, samples should be frozen (-20°C or lower).
Frozen or refrigerated samples must be brought to room temperature before testing. Use only pure aliquots for the test.
TEST PROCEDURE
The test should be performed at room temperature (10 °C to 30 °C)
1. Remove the test strip from the sealed package.
2. Immerse the strip in the urine so that the arrow points in the direction of the urine. Remove the strip after at least 10 seconds and place it horizontally on a clean, dry, non-absorbent surface (such as the neck of a urine container).
3. Evaluate the results after 5 minutes.
DO NOT interpret the test results later than 10 minutes.
IMPORTANT: Make sure that the urine level does not go beyond the MAX line (marking line), otherwise the test will not be performed correctly.
INTERPRETATION OF RESULTS
Positive (+)
A pale pink band is visualized in the test section. A colored band does not appear in the test site. A positive result indicates that the benzodiazepine concentration is at or above the detection limit (300 ng/mL).
Negative (-)
A pale pink band is visualized in the control and test areas. A negative result indicates that the benzodiazepine concentration is zero or below the limit of determination (300 ng/mL).
Invalid
If the stained strip is not visualized in the control area or if the stained strip is only visualized in the test area, the test results are invalid.
LIMITATIONS OF THE PROCEDURE
1. This test was developed for testing urine samples only. The efficacy of this test when using other samples has not been validated.
2. If tainted urine samples are used, erroneous results may be obtained. Strongly oxidizing agents such as bleach (hypochlorite) can oxidize drug samples. If a tampered sample is suspected, obtain a new sample.
3. This test is a qualitative screening test. It is not intended to quantify drug concentrations or intoxication levels.