|
Quantity
|
Out of stock
|
||
|
|
|||
The HIV1/2 (HIV1/2), Hepatitis C (HCV), Hepatitis B (HBsAg), Syphilis (TP) combination test is a rapid immunochromatographic assay for the qualitative detection of 4 immune markers, these: HIV1/2 (HIV1/2) antibodies, hepatitis c virus (HCV), hepatitis B surface antigen (HBsAg) and Treponema Pallidum (TP) antibodies in human whole blood/plasma/serum. For in vitro diagnosis only.
BRIEF DESCRIPTION
HIV1/2 (HIV1/2): HIV (human immunodeficiency virus) is the causative agent of AIDS (acquired immunodeficiency syndrome). HIV belongs to the retrovirus family of the lentivirus genus, and is divided into two groups HIV-1 and HIV-2. HIV-1 is highly mutagenic, and can be divided into 9 subtypes by membrane protein mutations (A, B, C, D, E, F, G, H and O). HIV-2 has 60% of the nucleotides homologous with HIV-1, but they differ in their ability to infect, HIV-1 being the predominant strain of the virus. Once infected, it mutates rapidly and has a poor prognosis. HIV-2 has a longer latency period, and is relatively weak in its pathogenesis.
Hepatitis C (HCV): Hepatitis C virus is an RNA virus that is a very active viral pathogen. Hepatitis C virus infection occurs despite a healthy immune system. It enters the body through direct contact with the blood. The virus attacks liver cells, where it multiplies (replicates). HCV causes inflammation of the liver and kills the liver cells. In 80%~85% of people pre-infected with HCV, the infection can go into a chronic form, meaning the infection does not appear for six months. Most people with chronic HCV have no symptoms. In 10% ~ 25% of people with chronic HCV, the disease progresses over 10 ~ 40 years and can lead to serious liver damage, cirrhosis and liver cancer.
Hepatitis B (HBV): Hepatitis B virus (HBV) belongs to the family hepadnaviridae and is a DNA-containing virus. Its nucleus contains circular double-stranded DNA and polymerases. Its envelope contains lipoproteins. There are five signal proteins in the blood and they are considered the main serologic hallmarks of HBV infection. These include hepatitis B surface antigen (HBsAg); hepatitis B surface antibody (HBsAb), hepatitis B envelope antigen (HBeAg), hepatitis B envelope antibody (HBeAb), and hepatitis B nuclear antibody (HBcAb). This test detects hepatitis B surface antigen (HBsAg).
Syphilis (TR): Syphilis is a curable infection caused by a bacterium called Treponema Pallidum (TP), which is acutely infectious. This infection is sexually transmitted and can also be passed from mother to fetus during pregnancy. The disease is spread mainly by sexual transmission or by close contact with a person who has an open, moist syphilitic ulcer.
More...
SPECIMEN COLLECTION AND PREPARATION
Whole blood collection:
1. Using standard blood collection procedures, collect a whole blood sample from a vein or finger using a blood collection tube.
2. It is recommended to test the samples at once. Do not leave samples at room temperature for an extended period of time. If samples are not tested immediately, they can be stored at 2°C ~ 8°C. Whole blood samples that have been stored at 2° C ~ 8° C for more than 7 days are not suitable for analysis.
Serum and plasma:
1. Using standard blood collection procedure, draw a whole blood sample from a vein using a blood collection tube. For plasma collection, use blood collection tubes with the appropriate anticoagulant.
Separate the serum/plasma as quickly as possible to avoid hemolysis.
3. The test should be performed immediately after specimen collection. Do not leave samples at room temperature for an extended period of time. Samples can be stored at 2°C ~ 8°C for up to 3 days. For long-term storage, samples should be stored at temperatures below -20°C.
Bring samples to room temperature before performing the test. Frozen samples should be completely thawed and well mixed before testing. Samples should not be refrozen and thawed repeatedly. Only clear, unhemalized samples can be used.
TEST PROCEDURE
Bring the device, buffer and sample to room temperature (10 °C ~ 30 °C) before performing the test.
1. Extract the test device with the sachet from the foil, tear at the tear line, and place it on a horizontal surface.
2. To analyze a whole blood sample:
① Holding the pipette vertically, add two drops of whole blood (approximately 50 µL) to each well for the test cassette sample. ② Add about two drops of buffer (included in the kit) from the vial directly into each sample well.
For serum/plasma sample analysis:
Holding the pipette vertically, add 3-4 drops (80µl ~ 100µl) of serum or plasma to each sample well (buffer solution is not used).
3. Wait 15 minutes and evaluate the results. Do not interpret the test results later than 30 minutes.
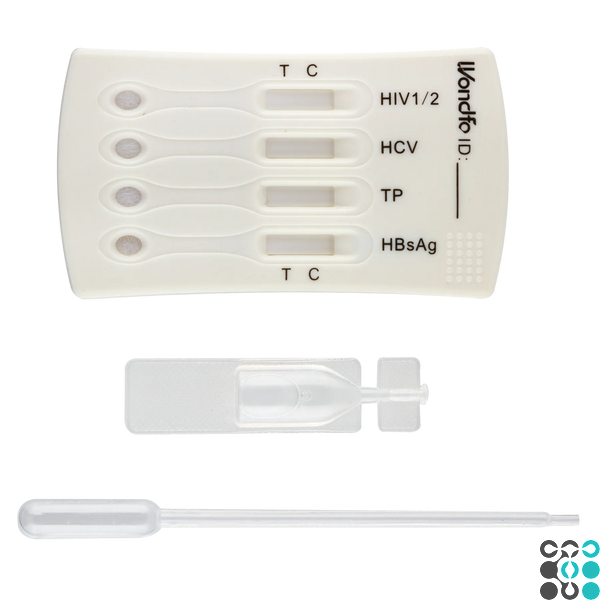
INTERPRETATION OF RESULTS
Positive (+)
Colored bars are visualized in both the control and test signs. This indicates a positive result.
Negative (-)
A colored band is only visualized in the control area. No colored band appears in the test region. This indicates that the antibody/antigen concentration in the sample is zero or below the detection limit of the test.
Invalid
There are no bands at all, or a band appears only in the test region and no band appears in the control region. Repeat the test using a new test kit.
LIMITATIONS OF THE PROCEDURE
1. This test has only been developed for testing whole blood/serum/plasma samples. The efficacy of this test in using other samples has not been confirmed.
2. As with any diagnostic procedure, confirmation of diagnosis can only be made after verification of all clinical and laboratory findings.
3. this test is a qualitative screening assay. It is not intended to quantify concentrations.
4. 4. If the test result is negative and clinical symptoms persist, additional testing by other clinical methods is recommended. A negative result does not exclude the possibility of infection.