|
Quantity
|
Out of stock
|
||
|
|
|||
The Wondfo whole blood/serum/plasma hepatitis C virus test cassette is a fast chromatographic immuno-test for the qualitative detection of hepatitis C virus antibodies.
Use only for in vitro diagnosis and only by healthcare professionals
Hepatitis C virus (HCV) is an RNA-containing virus with a very strong pathogenic factor. HCV has six major genotypes (subtypes): 1a, 1b, 2a, 2b, 3, 4, 5, and 6. Genotypes 1a and 1b are the most common and more difficult to treat.
It is possible to contract the hepatitis C virus despite a healthy immune system. The virus enters the body through blood contact. The virus attacks liver cells, where it multiplies (reproduces). HCV causes inflammation in the liver and kills liver cells. About 80%~85% of people who are infected with HCV develop a chronic infection, meaning the infection does not go away for six months. Most people with chronic hepatitis C have no symptoms. In 10%~25% of people with chronic hepatitis C, the disease progresses over 10~40 years and can lead to serious liver damage, cirrhosis and liver cancer.
More...
PRINCIPLE OF WORK
When a sample is introduced into the test device, it is absorbed into the pad by capillary forces, mixed with the antibody-dye conjugate and passed through the pre-treated membrane.
When HCV antibody levels are equal to or greater than the threshold values (sensitivity limit of the test), the HCV antibodies in the sample bind to the antibody-dye conjugate and are then fixed by the antigen immobilized in the test region (T) of the device. This results in a stained band, indicating a positive result.
If the HCV antibody levels are zero or below the sensitivity limit of the test, no colored band appears in the test region (T). This indicates a negative result.
If the test procedure is performed correctly, a colored band will appear in the control zone (C).
PRECAUTIONS
This test kit is for in vitro use only. Do not swallow.
2. All specimens should be considered as potential vector-borne disease agents.
Blood samples taken from jaundiced, lipemic patients; hemolyzed, heat-treated or contaminated blood samples may give erroneous results.
4. After the first use, the test system should be discarded. It can only be used once.
5. Do not use the test kit after the expiration date.
6. Do not use the test kit if the soft pack is damaged or poorly sealed.
7. Keep out of the reach of children.
8. DISPOSAL OF THE DIAGNOSTIC TEST: The used device is an infectious hazard. The disposal of the used test system should be done in accordance with local laws or laboratory regulations on infection.
STORAGE AND STABILITY
1. Store at 2°C ~ 30°C in an airtight soft pack until expiration date.
2. protect from direct sunlight, moisture. Keep away from heat sources.
3. DO NOT FREEZE.
COLLECTION AND PREPARATION OF SAMPLES
Use whole blood immediately, or separate serum or plasma from blood cells as soon as possible to avoid hemolysis. Only unadulterated and unhaemolysed samples can be used.
2 The test should be performed immediately after the samples have been collected. Do not leave samples at room temperature for long periods of time. Samples can be stored at 2○C~8○C for up to 3 days. For longer storage, samples should be stored at temperatures below -20°C. Whole blood samples that have been stored at 2° C -8° C for more than 7 days should not be tested.
3. Samples should be at room temperature (10ºC~30ºC) prior to testing. Frozen samples should be completely thawed and thoroughly mixed before the procedure. Samples should not be refrozen and thawed repeatedly.
TESTING PROCEDURE
Before testing, the unit and sample should be at room temperature (10°C ~30°C).
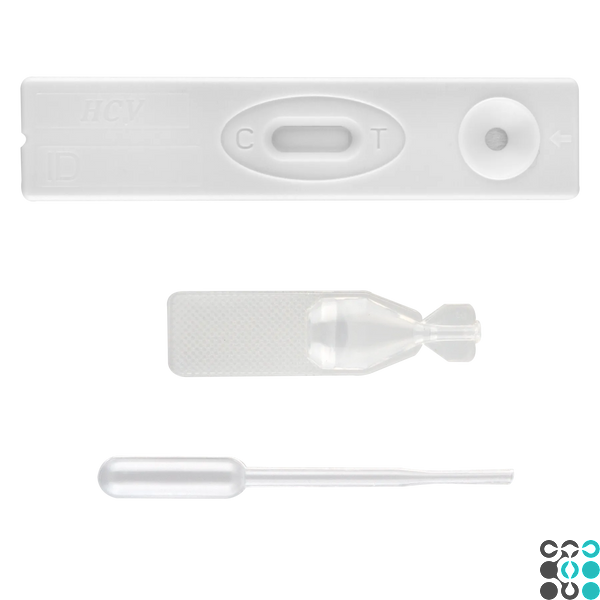
1. Take the test device out of the foil sachet by tearing it along the notch and place it on a flat surface.
2. For testing whole blood:
Holding the pipette vertically, add two drops of whole blood (approximately 50 µl) to the sample well (up to the mark line). Add two drops (approximately 50 µl) of buffered diluent directly from the vial to the sample well.
For testing serum/plasma:
Holding the sample pipette upright, add four drops (80-100 µL) of serum or plasma to the sample well.
Allow to stand for 15 minutes, then record the results. Do not read results after 30 minutes.
INTERPRETATION OF RESULTS
Positive (+)
Pale pink bands are visible in both the control and sample application areas. This indicates that HCV antibodies are positive in the sample.
Negative (-)
The pale pink band is visible in the test zone. No colored band appears in the test zone. This indicates that the concentration of HCV antibodies is zero or less than the sensitivity threshold of the test system.
Invalid
No bands are visible, or there is such a band only in the test area but not in the control area. Repeat the procedure with a new test kit. If the test fails, contact the distributor or store where you purchased the product with the lot number.
Note: Color intensity or line width does not matter.
LIMITATIONS OF THE TEST PROCEDURE
1 This test is designed to test whole blood/serum/plasma samples only. There is no evidence that this test system will work with other samples.
2. This test is a qualitative immunological screening procedure. It is not intended to quantify HCV antibody concentrations.