|
Quantity
|
Out of stock
|
||
|
|
|||
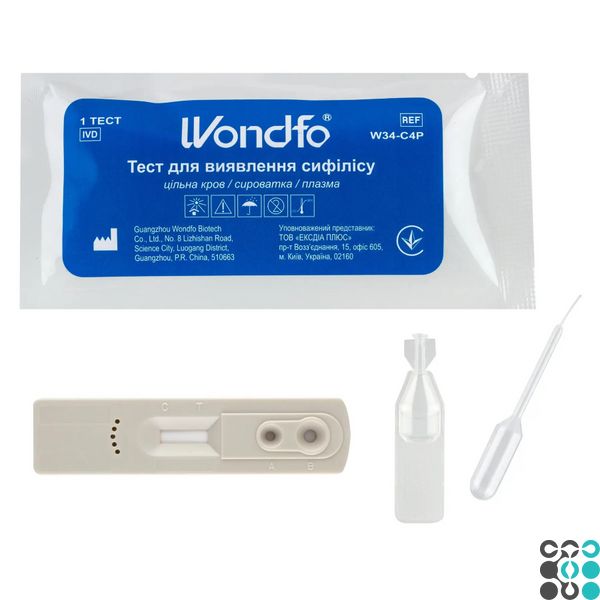
The Wondfo test cassette for the detection of syphilis in whole blood/serum/plasma is a rapid test for the visual detection of antibodies to pale spirochete (Treponema pallidum) as an aid in the diagnosis of syphilis infection.
Use only for in vitro diagnosis and only by health care professionals.
Syphilis is a sexually transmitted disease (STD) that is highly infectious. The disease is spread mainly by sexual contact or direct contact with a person who has an open, moist syphilitic ulcer. Syphilis has three distinctive stages of neglect in humans. This most severe STD is caused by a pathogen called the pale spirochete Treponema pallidum (TP), which is corkscrew-shaped so that it can break through the skin quite well and eventually enter almost anywhere in the body. The ulcer marks the spot where the syphilis pathogen penetrates the skin and body. Within a few weeks, fever, chills, aches, headache and swollen tonsils develop. Sometimes there is also a rash. The second stage is called the latent period. This is where the spirochetes enter the bloodstream, usually six to eight weeks after the ulcer chancre appears. At first, there may be no symptoms. At this stage, the person is no longer contagious, or unable to spread the disease, but still sick. The most distinctive feature of the second stage is the appearance of a rash. If left untreated, it progresses to the third stage of the disease, where brain damage can occur, as well as blindness, paralysis and disorientation, and damage to blood vessels, which contributes to clots and aneurysms. Most people do not reach the above stages because there is currently a treatment available. Thus, the Wondfo test system for syphilis can quickly help in the early diagnosis and timely treatment of the disease.
More...
PRINCIPLE OF OPERATION
The Wondfo test system for detection of syphilis in whole blood/serum/plasma is based on the sandwich method of dual antigen immunoassay for detection of syphilis antibodies in whole blood/serum/plasma. The recombinant TpN15, TpN17 and TpN47 antigens are coated with a solid membrane. There are two coated lines in the results window. One is the test line (T) coated with recombinant antigens and the other is the control line (C) coated with polyclonal antibodies. When the sample is introduced into the well, it is absorbed into the device by capillary forces, mixed with the antigen-dye conjugate and passed through the pre-coated membrane.
When the levels of syphilis antibodies in the sample meet or exceed threshold values (test sensitivity limit), the syphilis antibodies bind to the antigen-dye conjugate and are then captured by the recombinant antigen immobilized in the test region (T) of the device to form Ag-Ab-Ag-Au precipitates. This results in a stained band in the test region, indicating a positive result.
When syphilis antibody levels are zero or below thresholds, no colored band appears in the test region T, indicating a negative result.
If the test procedure is performed correctly, a colored line will appear in the control zone (C).
PRECAUTIONS
This test kit is for in vitro use only. Do not swallow.
2. All specimens should be considered as vector-borne disease agents.
3. Blood samples taken from jaundiced, lipemic patients; hemolyzed, heat-treated or contaminated blood samples may give erroneous results.
4. After the first use, the test system should be discarded. It can only be used once.
5. Do not use the test kit after the expiration date.
6. Do not use the test kit if the soft pack is damaged or poorly sealed.
7. Keep out of the reach of children.
8. DISPOSAL OF THE DIAGNOSTIC TEST: The used device is an infectious hazard. The disposal of the used test system should be done in accordance with local laws or laboratory regulations on infection.
STORAGE AND STABILITY
1. Store at 2°C ~ 30°C in an airtight soft pack until expiration date.
2. protect from direct sunlight, moisture. Keep away from heat sources.
3. DO NOT FREEZE.
COLLECTION AND PREPARATION OF SAMPLES
Whole blood taken from a finger:
1. Choose a finger to pierce, usually on the side of the ring finger. Wipe the puncture area with an alcohol wipe. Allow the finger to dry.
Using a sterile lancet, puncture the skin near the center of the fingertip. Hold your finger down. Apply light pressure near the puncture point. Do not squeeze your finger to let the blood drain out. Wipe off the first drop of blood with a sterile swab. Let a new drop of blood form. If the blood flow is insufficient, the patient's finger can be lightly massaged at the base to get a sufficient volume drop. Do not "milk" the finger.
3. Touch the capillary end of the blood drop to draw approximately 10 µl. Avoid the accumulation of air bubbles. Whole blood samples collected from the finger should be used immediately after collection.
Whole blood collected from a vein:
1.Using standard blood draw procedure, collect whole blood samples from the vein into blood collection tubes. When collecting plasma, use blood collection tubes containing a suitable anticoagulant.
2. It is recommended that samples be tested immediately. Do not leave samples at room temperature for extended periods of time. If samples are not tested immediately, they can be stored at 2°C~8°C. Do not test whole blood samples that have been stored at 2°C~8°C for more than 7 days.
Serum and plasma:
1. Using standard blood draw procedure, collect whole blood samples from the vein into blood collection tubes. When collecting plasma, use blood collection tubes containing a suitable anticoagulant.
Separate serum/plasma from blood cells as soon as possible to avoid hemolysis.
3. The test should be performed immediately after specimen collection. Do not leave samples at room temperature for extended periods of time. Samples can be stored at 2○C~8○C for up to 3 days. For longer storage, samples should be stored below -20°C.
Samples should be taken to room temperature before testing. Frozen samples should be completely thawed and thoroughly mixed before the procedure. Samples should not be repeatedly frozen and thawed. Only specimens without impurities, without hemolysis, can be used
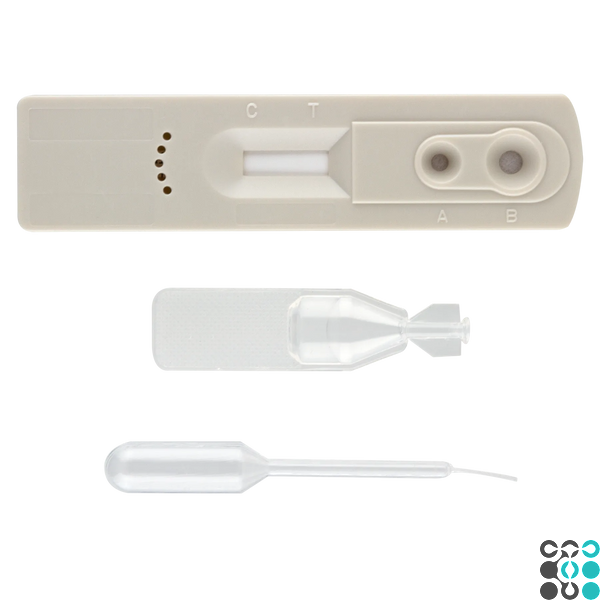
TESTING PROCEDURE
The instrument, buffer, and sample must reach room temperature (10°C ~30°C) before testing.
Remove the test cassette from the foil sachet by tearing it open along the notch and place it on a flat surface.
2. Slowly add 10 µl (second marker line) of whole blood or serum or plasma to the sample well (A), then add 2 drops of dilution buffer to the buffer well (B).
3. At the beginning of the testing process, you will see the magenta colored line move in the results window in the center of the test device.
4. Leave it for 15 minutes and then record the results. Do not read the results after 30 minutes.
INTERPRETATION OF RESULTS
Positive (+)
Stained bands are visible in both the control area and the sample application area. This indicates a positive result for the antibody to TR in the sample.
Negative (-)
The colored band is only visible in the control zone. No colored band appears in the sample application area. This indicates that the concentration of antibodies to the TR of the sample is zero, or it is below the sensitivity threshold of the test system.
Invalid
No visible bands, or such a band appears only in the sample application area but not in the control area. Repeat the procedure with a new test kit. If test fails, contact Wondfo or distributor for technical assistance.
Note: Color intensity or line width is not important.
LIMITATIONS OF THE TEST PROCEDURE
- This test is designed to test only whole blood/serum/plasma samples.
- As with any other diagnostic procedure, the results of this test can only be considered in conjunction with the rest of the clinical and laboratory data.
- This test is a qualitative immunologic screening procedure. It is not intended to quantify the concentration of antibodies to syphilis.
- If the test result is negative and clinical symptoms persist, additional investigations by other clinical methods are recommended. A negative result does not rule out the possibility of syphilis infection.