|
Quantity
|
Out of stock
|
||
|
|
|||
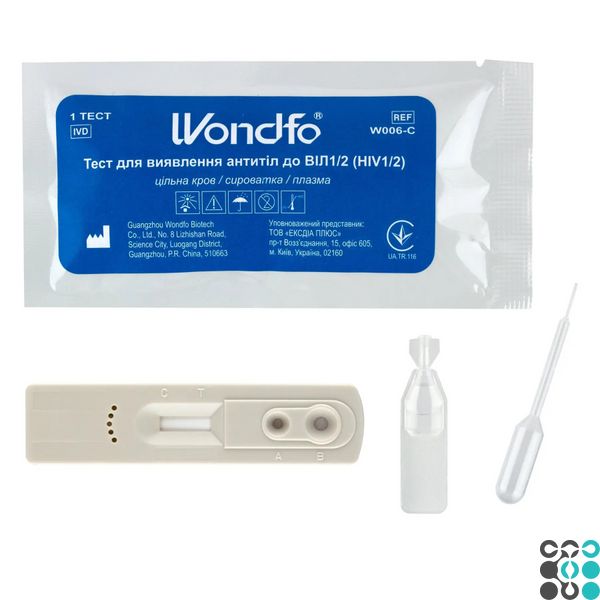
The Wondfo test cassette for the detection of HIV type 1/2 (2 lines) in whole blood/serum/plasma, is a rapid one-step test for the qualitative detection of antibodies at certain thresholds.
Use only for in vitro diagnosis and only by healthcare professionals.
HIV (human immunodeficiency virus) is the causative agent of AIDS (acquired immunodeficiency syndrome). HIV belongs to the retrovirus family, a genus of lentiviruses; there are two types of viruses, HIV-1 and HIV-2. HIV-1 is highly mutagenic and can be subdivided into 9 subtypes based on membrane protein mutations: A, B, C, D, E, F, G, H and O. HIV-2 has 60% homology with HIV-1 in its RNA, but they differ in their ability to infect, HIV-1 being the most predominant strain. Once infected, it mutates rapidly and the infected person has a poor prognosis. HIV-2 has a longer latency period and its pathogenesis is relatively weaker.
More...
PRINCIPLE OF WORK
When a sample is introduced into the test device, it is absorbed by the liner by capillary forces, mixed with the antibody-dye conjugate, and passed through the pre-treated membrane.
When HIV antibody levels equal or exceed the sensitivity limit of the test, the HIV antibodies in the sample bind to the antibody-dye conjugate and are then fixed by the antigen immobilized in the test region (T) of the device. This results in a colored band, indicating a positive result.
If the HIV antibody levels are zero or below the sensitivity limit of the test, no colored band appears in the test zone T. This indicates a negative result.
If the test procedure is performed correctly, a colored band will appear in the control zone (C).
PRECAUTIONS
This test kit is for in vitro use only. Do not swallow.
2. All samples should be considered as potential infectious diseases.
3. Blood samples taken from jaundiced, lipemic patients; hemolyzed, heat-treated or contaminated samples may give erroneous results.
4. After the first use, the test system should be discarded. It can only be used once.
5. Do not use the test kit after the expiration date.
6. Do not use the test kit if the soft pack is damaged or poorly sealed.
7. Keep out of reach of children.
8. DISPOSAL OF THE DIAGNOSTIC TEST: The used device is an infectious hazard. The disposal process of the used test system should be carried out in accordance with local laws or laboratory regulations on infection.
STORAGE AND STABILITY
1. Store at 2°C ~ 30°C in an airtight soft pack until expiration date.
2. protect from direct sunlight, moisture. Keep away from heat sources.
3. DO NOT FREEZE.
COLLECTION AND PREPARATION OF SAMPLES
Whole blood taken from a finger:
1. Select the finger to pierce, usually on the side of the ring finger. Wipe the puncture area with an alcohol wipe. Allow the finger to dry.
Using a sterile lancet, puncture the skin near the center of the fingertip. Hold your finger down. Apply light pressure near the puncture point. Do not squeeze your finger to let the blood drain out. Wipe off the first drop of blood with a sterile swab. Let a new drop of blood form. If the blood flow is insufficient, the patient's finger can be lightly massaged at the base to get a sufficient volume drop. Do not "milk" the finger.
3. Take the specimen pipette, squeeze lightly, dip the open end into the blood drop, and then, without applying pressure, draw the blood into the pipette. Whole blood samples collected from the finger should be used immediately after collection.
Whole blood collected from a vein:
1. Using standard blood draw procedure, collect whole blood samples from the vein into blood collection tubes. When collecting plasma, use blood collection tubes containing a suitable anticoagulant.
2. It is recommended that samples be tested immediately. Do not leave samples at room temperature for extended periods of time. If samples are not tested immediately, they can be stored at 2°C~8°C. Do not test whole blood samples that have been stored at 2°C~8°C for more than 7 days.
Serum and plasma:
Using standard blood draw procedure, collect whole blood samples from a vein into blood collection tubes. When collecting plasma, use blood collection tubes containing a suitable anticoagulant.
Separate serum/plasma from blood cells as soon as possible to avoid hemolysis.
3. The test should be performed immediately after specimen collection. Do not leave samples at room temperature for extended periods of time. Samples can be stored at 2○C~8○C for up to 3 days. For longer storage, samples should be stored below -20°C.
Samples should be taken to room temperature before testing. Frozen samples should be completely thawed and thoroughly mixed before the procedure. Samples should not be repeatedly frozen and thawed. Only specimens without impurities, without hemolysis, can be used
TESTING PROCEDURE
The instrument, buffer, and sample must reach room temperature (10°C ~30°C) before testing.
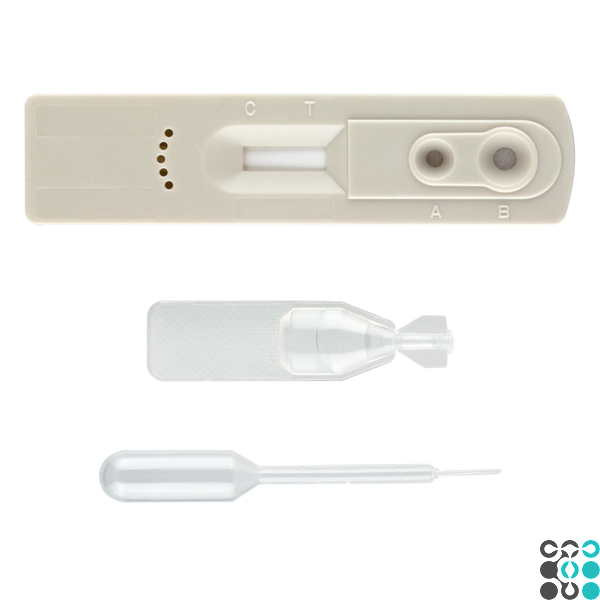
1. Remove the test cassette from the foil sachet by tearing it along the notch and place it on a flat surface.
2. Slowly add 10 µl (second marker line) of serum or plasma into the sample well (A), then add 2 drops of dilution buffer into the buffer well (B).
3. At the beginning of the testing process, you will see the magenta colored line move in the results window in the center of the test device.
4. Leave it for 15 minutes and then record the results. Do not read the results after 30 minutes.
INTERPRETATION OF RESULTS
Positive (+)
Pale pink bands are visible in both the control and sample application areas. A positive result indicates that the presence of HIV 1/2 is equal to or greater than the sensitivity threshold of the test system.
Negative (-)
A pale pink band is visible in the control zone. No colored band appears in the test zone. This indicates that the concentration of HIV 1/2 antibodies is zero, or is below the sensitivity threshold of the test system.
Invalid
No bands are visible, or there is such a band only in the test zone and not in the control zone. Repeat the procedure with a new test kit. If the test fails, contact the distributor or store where you purchased the product with the lot number.
Note: Color intensity or line width does not matter.
LIMITATIONS OF THE TEST PROCEDURE
This test is designed to test whole blood/serum/plasma samples only.
2. This test is a qualitative immunological screening procedure. It is not designed to quantify HIV concentrations.
Negative result does not exclude HIV infection, as antibodies to HIV may not be present or may not be present in sufficient quantities to allow their detection at an early stage of infection.
4. 4. If positive, verify this by using ELISA or Western Blotting.